People living with HIV (PLHIV), health workers, academics, researchers, and treatment providers agree that one of the biggest programmatic challenges of the HIV response today is how programmes should address treatment failure, delayed initiation, and treatment interruption: all of which lead to advanced HIV disease. Severe bacterial and fungal infections are – next to TB – the leading causes of morbidity and mortality among PLHIV.
Civil society has been battling for many years for increased access to liposomal amphotericin B (L-AmB), an antifungal medicine produced by the American pharmaceutical corporation, Gilead As a result of Gilead’s monopoly control of the price as well as supply through both exclusive sourcing of components as well as being the only company to achieve WHO requirements, we’re now facing a situation of shortage of L-AmB. L-AmB is used (together with other essential antifungals, such as flucytosine and fluconazole) to treat cryptococcal meningitis (CM), a fungal brain infection that particularly affects PLHIV.
After all these years, these medicines are still only available in a handful of countries – and where they are available, they are often unaffordable. Some of these medicines are also used for other neglected diseases such as kala- azar. Now there is even more pressure on their global supply, especially for L-AmB, given the urgent needs of people affected by COVID-19 and its associated black fungus (mucormycosis) outbreak in India and Nepal.
Cryptococcal Disease and Treatment Access: FAQs and key messages to Gilead
Cryptococcal meningitis is a painful opportunistic infection, most commonly affecting people living with advanced HIV. Effective treatments exist and rely on three key medicines: fluconazole, flucytosine and liposomal amphotericin B (L-AmB). Unfortunately, for several reasons, L-AmB is not as widely accessible as needed. As an international medical humanitarian organisation, Médecins Sans Frontières (MSF) relies on access to L-AmB to treat cryptococcal meningitis and other diseases. Like many other treatment providers, MSF has experienced challenges accessing affordable L-AmB.
These frequently asked questions explain what cryptococcal meningitis is, why L-AmB is needed for treatment, what the access challenges are, and what Gilead should do to improve access as the manufacturer of the only quality-assured version of L-AmB currently available.
What is advanced HIV and how is it diagnosed?
People living with HIV are still dying of AIDS, also referred to as advanced HIV. World Health Organization (WHO) 2017 guidelines on managing advanced HIV disease state the following criteria for people living with HIV to be considered to have advanced HIV:(1)
For adults, adolescents, and children five years or older, advanced HIV is defined as a CD4 cell count <200 cells/mm3 or a WHO clinical stage (3 or 4) diagnosis at presentation for care. All children with HIV who are younger than five years old should be considered as having advanced disease at presentation for care.
CD4 testing is needed to diagnose advanced HIV, yet people in 12 African countries have limited or no access to routine CD4 testing.(2)
What is cryptococcal meningitis?
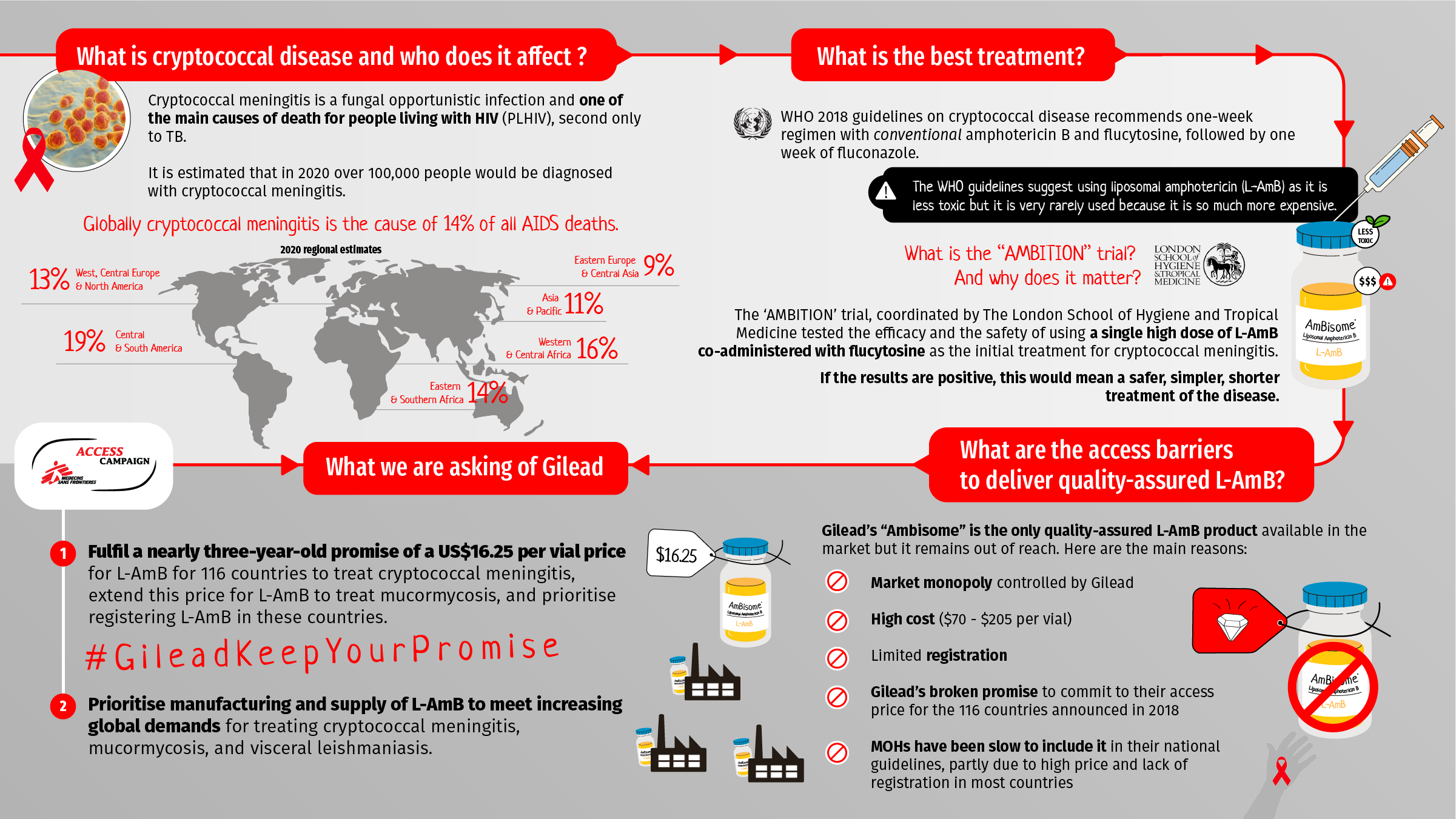
Cryptococcal meningitis is a painful fungal infection of the brain and surrounding membranes occurring primarily among people with advanced HIV (as an ‘opportunistic infection’). Responsible for about 14% of AIDS-related deaths worldwide, it is second only to tuberculosis as a cause of death for people living with HIV.(3)
What is the global burden of cryptococcal meningitis?
While Sub-Saharan African countries have the highest burden of cryptococcal meningitis, it is also common in those with advanced HIV in Asia and is responsible for the largest proportion of AIDS related deaths in Central and South America.
Among people living with HIV globally in 2020, an estimated 174,500 had the cryptococcal antigen (CrAg+), an estimated 108,000 of whom had cryptococcal meningitis.(3)
What are the global guidelines on screening for cryptococcal disease?
The WHO 2017 advanced HIV disease guidelines and 2018 guidelines on cryptococcal disease recommend that everyone with advanced HIV should be screened for cryptococcal antigen using the cryptococcal antigen test.(1,4)
When cryptococcal antigen screening is positive, people should ideally proceed to have a lumbar puncture to determine whether full or pre-emptive treatment is needed, as recommended in the WHO 2018 cryptococcal disease guidelines.(4)
What are the global guidelines on prevention of cryptococcal disease?
When cryptococcal antigen screening is not available, fluconazole primary prophylaxis should be given to adults and adolescents living with HIV who have a CD4 cell count <100 cells/mm3 and may be considered for those with a CD4 cell count <200 cells/mm. (3) Similarly, if after a positive cryptococcal antigen test the next step of a lumbar puncture is negative (or not available), fluconazole is recommended for pre-emptive treatment.
What are the global guidelines on treatment of cryptococcal disease?
Cryptococcal disease can be treated with amphotericin B, which is available in conventional and liposomal formulations. Studies have shown conventional and liposomal amphotericin B to have equivalent efficacy at treating cryptococcal meningitis.(5,6) However, the liposomal formulation is less toxic (especially for the kidneys) and therefore easier to give to people needing treatment, especially in settings with limited capacity for monitoring.
The WHO 2018 guidelines on cryptococcal disease recommend a one-week regimen with conventional amphotericin B and flucytosine, followed by one week of fluconazole.(4) One of the reasons that WHO does not insist on liposomal amphotericin B (L-AmB) in its recommendations is the difficulty in procuring it and the high price.
What is the AMBITION trial?
The AMBITION trial, coordinated by The London School of Hygiene and Tropical Medicine, tested the efficacy and the safety of using a single high dose of L-AmB co-administered with flucytosine as the initial treatment for cryptococcal meningitis.
Participants were recruited in African collaborating institutions in conjunction with local hospitals in the following countries: Botswana, Malawi, South Africa, Uganda and Zimbabwe.
The results of the AMBITION trial will be presented at the 2021 International AIDS Society conference. If the results are positive, the regimen would be an opportunity to make cryptococcal meningitis treatment simpler and safer, potentially reducing the number of days required for inpatient admission and reducing the overall costs of treating cryptococcal meningitis.
What are the barriers preventing people with cryptococcal meningitis from accessing L-AmB?
Gilead’s L-AmB product, marketed as Ambisome, is the only quality-assured L-AmB product available in the market.
Although generic companies have been working for years to develop L-AmB, they face multiple hurdles. L-AmB is not patented, but Gilead has long hidden the liposomal technology – a key component of manufacturing L-AmB – as a trade secret. This, combined with limited availability of raw materials and challenging regulatory pathways, has significantly delayed generic competition.
The absence of generic manufacturers has resulted in a lack of sustainable supply of L-AmB. Access to L-AmB also remains extremely limited in low- and middle-income countries (LMICs) for several additional reasons:
• Gilead has failed to provide sufficient access to the treatment at the ‘access price’ of US$16.25 promised for 116 countries, as announced in September 2018.(7) For example, although India and South Africa are on the list of the countries eligible for Gilead’s access price, the treatment is not easily available at that price in either country. Gilead is not the market authorisation holder in these countries. Instead they signed exclusive license distribution agreements with local suppliers, leaving these countries’ prices set by Gilead’s marketing partners. The price of L-AmB in these markets can range between $70-205 per vial,(8) and local suppliers are reluctant to fulfil Gilead’s obligation to supply L-AmB at the access price.(9)
•The price of L-AmB is high in the private market in LMICs ($70-200 per vial). • Gilead has failed to register L-AmB in some of the 116 countries eligible for the access price announced in September 2018.(b)
• There is insufficient funding for cryptococcal meningitis treatment programmes because it is neglected, and not always prioritised by donors and governments due to high prices.
• Due to unaffordable prices or limited availability because of a lack of registration, L-AmB is inaccessible for most countries with high burdens of cryptococcal meningitis. Many ministries of health have not included it into their national guidelines for treatment of cryptococcal meningitis.
Is there a generic version of L-AmB in the pipeline?
MSF has tried several times to find a generic source of L-AmB. Some generic companies registered the product but never put it into production. Other generic sources faced quality assurance challenges due to a lack of appropriate regulatory guidance from WHO and national medicines regulatory authorities (NMRAs). The mucormycosis outbreak in India and increased demand for generic versions of L-AmB has led to generic companies entering the supply chain and approaching India’s NMRA for approval. These new sources are yet to be WHO pre-qualified and made available for supply to other countries. Many public health actors are working to address both WHO prequalification and international supply and looking into how a generic source of L-AmB can be made available as a long term solution.
What does Gilead need to do to improve access to this lifesaving treatment?
As the supplier of the only quality-assured L-AmB product currently available, two actions Gilead should immediately take to improve access to L-AmB are:
• Fulfil a nearly three-year-old promise of a $16.25 per vial price for L-AmB for 116 countries to treat cryptococcal meningitis, extend this price for L-AmB to treat mucormycosis, and prioritise registering L-AmB in these countries; and
• Prioritise manufacturing and supply of L-AmB to meet increasing global demands for mucormycosis treatment, ongoing needs for visceral leishmaniasis treatment, and cryptococcal meningitis treatment needs.
References:
1 WHO. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. [Online]. 2017 [Cited 2021 Jul 12]. Available from: https://apps.who.int/iris/bitstream/handle/10665/255884/9789241550062-eng.pdf?sequence=1 2 Ending Cryptococcal Meningitis Deaths by 2030 – Strategic Framework. South Africa. [Online]. 2021 [Cited 2021 Jul 12]. Available from: https://msfaccess.org/sites/default/files/2021-05/Cryptococcal%20Meningitis_Briefing_Doc-END_CM%20DEATHS_2030- strategic%20framework_ENG_14.5.2021.pdf
3 Rajasingham R. The global burden of HIV-associated cryptococcal infection: 2020 edition. [Powerpoint presentation]. Ending Cryptococcal Meningitis Deaths by 2030 – A New Global Initiative. Advanced HIV Disease Series. 2021 May 12 [Cited 2021 Jul 12]. Available from: https://iecho.unm.edu/sites/unm/download.hns?i=30835
4 WHO. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. [Online]. 2018 [Cited 2021 Jul 21]. Available from: http://apps.who.int/iris/bitstream/handle/10665/260399/9789241550277- eng.pdf;jsessionid=3DD47D6B4F06AFA32280E2048900A316?sequence=1
5 Leenders A, Reiss P, Portegies P, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. [Online]. 1997 [Cited 2021 Jul 13]; 11(12). Available from: https://pubmed.ncbi.nlm.nih.gov/9342068/
6 Hamill R, Sobel J, El-Sadr W, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis. [Online]. 2010 [Cited 2021 Jul 13]; 51(2). Available from: https://pubmed.ncbi.nlm.nih.gov/20536366/
7 Gilead Sciences announces steep discounts for Ambisome to treat cryptococcal meningitis in low- and middle-income countries. Press release. [Online]. 2018 Sep 7 [cited 2021 Jul 8]. Available from: https://www.gilead.com/news-and-press/company-statements/discount for-ambisome
8 Medicine Prices. What should your medicines cost? [Online]. 2020 [Cited 2021 Jul 12]. Available from: https://medicineprices.org.za/#search:amphotericin
9 MSF. Untangling the web: HIV medicine pricing and access issues, 2020. [Online]. 2020 [Cited 2021 Jul 12]. Available from: https://msfaccess.org/sites/default/files/2020-11/HIV_Brief_Untangling-the-Web_2020.pdf

